Calcium is one of the most crucial macronutrients essential for plants. Its content in the Earth’s crust is approximately 3.6% of its mass, significantly higher compared to other macronutrients such as potassium, magnesium, and phosphorus. Calcium is the dominant cation in both the sorption complex and soil solution of arable soils. About 60-80% of its total capacity in the soil is interchangeably bound by the sorption complex. The primary source of calcium in the soil is limestone minerals such as hornblende, calcite, gypsum, phosphate, and dolomite. The final calcium content in the soil is mainly determined by the type of parent rock from which the soil originated and the degree of weathering and leaching processes (Juo and Barber 1969, Barber 1995, Wójcik 1998).
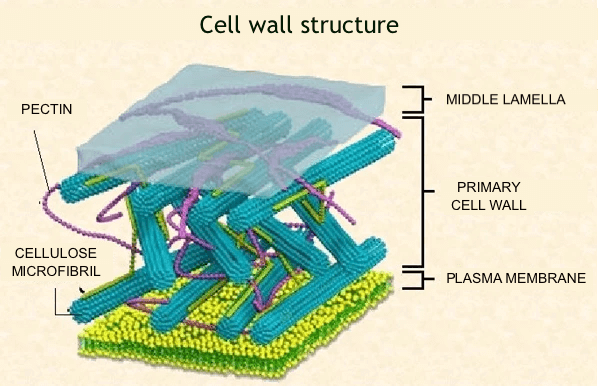
The most common calcium content in the soil solution is 200-300 mg Ca2+∙dm-3, considered optimal for most cultivated crops. However, for vegetable cultivation, especially for cabbage, the soil’s calcium content should be higher, ranging from 800 mg Ca2+∙dm-3 to 2000 mg Ca2+∙dm-3 for celery. The uptake of calcium ions (Ca2+) by plants is largely genetically determined, with monocots generally taking smaller amounts compared to most dicots (Bousquet et al. 1981; Wójcik 1998; Marschner 2012). Calcium ions, like other mineral components, are taken up by roots along with water. In field conditions, insufficient soil moisture is a decisive factor limiting the uptake of mineral nutrients by plants (Shear 1975; Wójcik 1998). Water deficiency in the soil strongly reduces the movement of calcium ions towards the root surface because plant transpiration is limited (Barber 1995). The calcium absorbed by the roots is primarily transported to the aboveground parts of the plant through the xylem. Approximately 60% of the total calcium content in the plant is stored in the cell wall, where it forms complexes with cellulose and pectins, including calcium pectinate in the middle lamella (Figure 1).
Calcium also serves as a cofactor for enzymes such as ATPases, phospholipases, and amylases. Its role during mitotic division in plant meristems is crucial for root system development and the formation of root hairs, laying the foundation for future yields. Too low levels of Ca2+ can block anaphase, inhibiting plant growth. Calcium deficiency can disrupt the growth of corn pollen tubes, leading to incomplete kernel fertilization. Organs with generative functions, such as seeds and fruits, often exhibit low calcium concentrations. Calcium ions, along with potassium (K+), regulate plant water balance by controlling transpiration, and the opening and closing of leaf stomata. Plants adequately supplied with calcium, which controls the water photolysis process, stay green and photosynthetically active for a longer period, resulting in increased yield.
Besides the type of parent rock and the advancement of weathering and leaching processes, lime fertilizers are another significant source of calcium for plants. Their application not only meets plant nutritional needs but also helps regulate soil pH. Soil acidification is a natural process associated with Poland’s geographical location. Natural and anthropogenic processes cause annual calcium losses in the soil, reaching at least 140 kg CaO·ha-1, and in intensively cultivated and fertilized areas, especially with nitrogen, and in heavily polluted regions, exceeding 250 kg CaO·ha-1.
Soil acidification is influenced by:
a) leaching of lime by precipitation, approximately 100-150 kg Ca∙ha-1, sometimes exceeding 200 kg CaO·ha-1 for soils freshly limed with large lime doses at pH above 6.5;
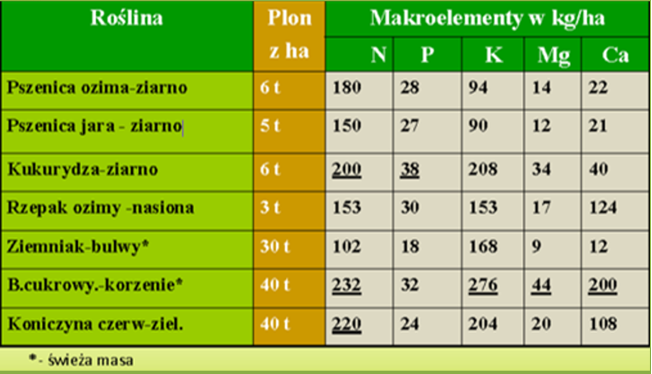
b) removal of calcium with crop yield, from 12 kg CaO·ha-1 for potatoes to 200 kg CaO·ha-1 for sugar beets (Table 1).
c) consumption of calcium to neutralize so-called acid rain, 20-40 kg CaO·ha-1;
d) use of mineral fertilizers, especially nitrogenous ones, as neutralizing 1 kg N acidifying action requires at least 1.0-1.5 kg CaO, and sulfuric ones, where 1 kg S neutralization requires a minimum of 2 kg CaO.
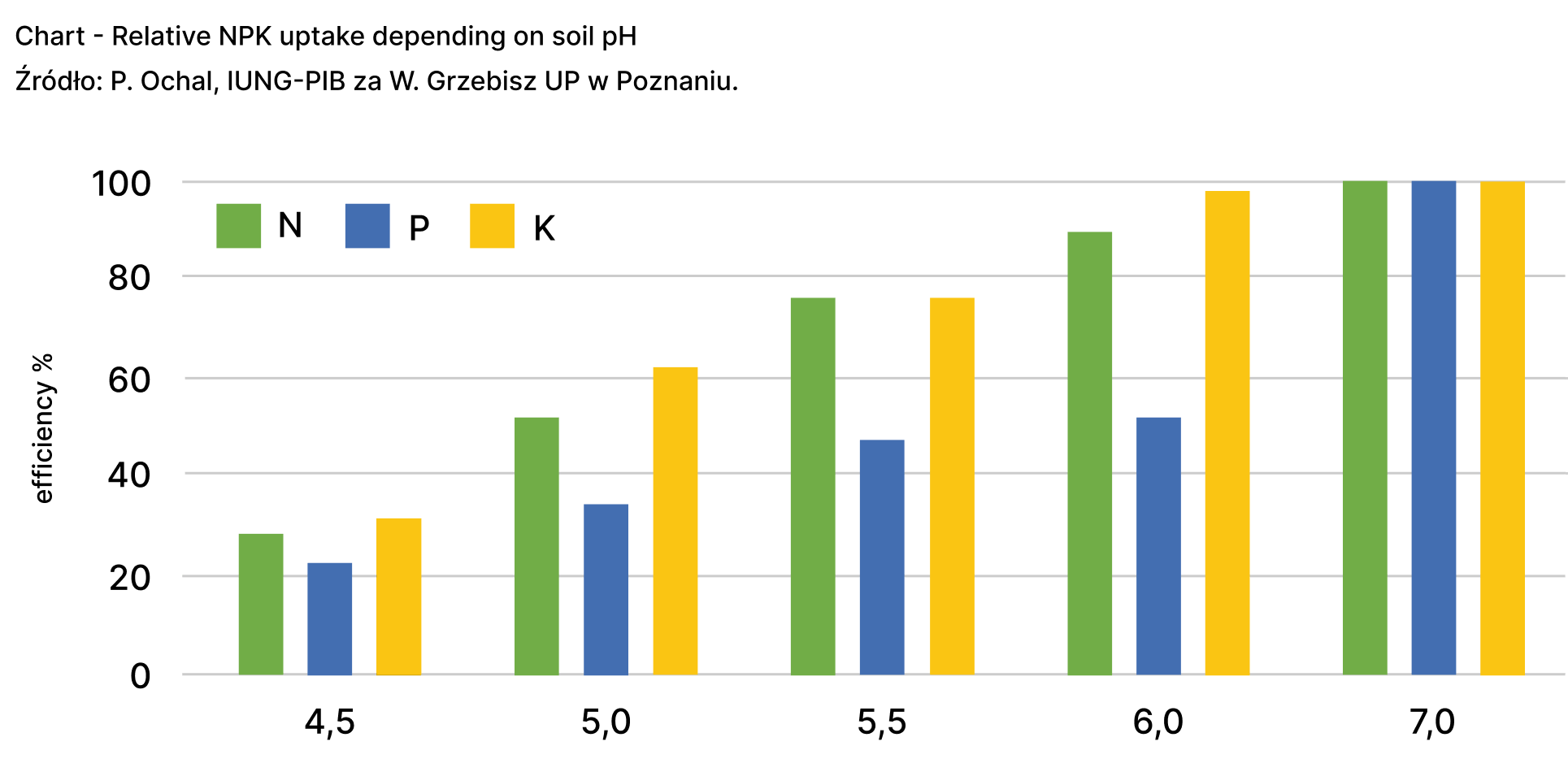
It is estimated that about 73% of Poland’s agricultural land, approximately 9,394,429.9 hectares, is to some extent acidified. This includes strongly acid soils (16%), acid soils (26%), and slightly acid soils (31%). The majority of very acid and acid soils (about 58-63%), requiring significant lime fertilizer doses, are found in the Łódź (60%), Mazovia (58%), Subcarpathian, and Podlaskie (62%) voivodeships (Ochal et al. 2017, Statistical Yearbook of Agriculture 2022). Soil pH, indicated by the value of pH, is a fundamental and easily measurable indicator of fertility. Soils with a pH of 5.0 or lower increase aluminum mobility, inhibiting root growth and acting phytotoxically on root hairs, causing them to die. This negatively affects the transport of water and mineral salts from the soil solution to the aboveground parts of plants and reduces the efficiency of NPK utilization by crops (Figure 2).
In addition, plant roots are more susceptible to infection, especially by pathogens. A high concentration of aluminum in the soil solution hampers the uptake and transport of calcium and magnesium. Acidified soils generally have increased manganese concentrations, very low or low phosphorus content, and often low potassium content available for plants. Phosphorus deficiency makes it impossible to achieve high yields, even with large doses of nitrogen fertilizer. Very acidic and acidic soils usually show low and very low available potassium content. Plants grown on such soils suffer from magnesium deficiency, leading to growth inhibition and, in severe cases, plant death. Plants on acidified soils have lower resistance to drought, frost, and diseases and pests. Agricultural soils should ideally have a pH value between 5.5 and 7.0, with a lower value for light soils and a higher one for heavy soils. The only effective way to regulate soil pH is through liming (Kopiński et al. 2013; Krasowicz et al. 2011; Ochal 2012).
When planning liming treatments in crop rotations, it is essential to remember that different crops have varying soil pH requirements. Crops such as wheat, barley, corn, sugar beets, rapeseed, soybeans, and others that respond favorably to soil pH (from 6.0 to 7.5) benefit from lime fertilizers such as dusty lime, e.g., Kujawit or Radkowit (lime with magnesium) applied before the preceding crop. If, for various reasons, lime was not applied during this period, the post-harvest period is another suitable time for soil liming. This timing allows thorough mixing of the applied lime with the soil and provides a longer period for soil pH stabilization before sowing or planting crops. According to Pietr and Krysztoforski (2022), the commonly used liming model once every 3-4 years is inappropriate because it leads to sinusoidal fluctuations in soil pH, causing biological, chemical, and physical disruptions in soil functions. Maintaining a stable soil pH, at least pH 6.0, ensures effective utilization of mineral nutrients, and proper development of the root system, and contributes to the correct development of soil microorganisms responsible, among other things, for increasing organic matter content in the soil (Pietr and Krysztoforski 2022).
If the period between the harvest of the preceding crop and the sowing of a crop highly sensitive to soil pH is relatively short, it is recommended to use granulated carbonate lime, e.g., Polcalc III Generation, especially after crop emergence (Figure 3).